AVIAN INFLUENZA- A REVIEW
Definition
Avian influenza (AI) is a disease of viral etiology that ranges from a mild
or even asymptomatic infection to an acute, fatal disease of chickens,
turkeys, guinea fowls, and other avian species, especially migratory
waterfowl
Etiology
Fowl plague was described in 1878 as a serious disease of chickens in Italy.
It was determined in 1955 that fowl plague (FP) virus is actually one of the
influenza viruses. The AI viruses, along with the other influenza viruses,
make up the virus family Orthomyxoviridae. The virus particle has an
envelope with glycoprotein projections with hemagglutinating and
neuraminidase activity. These two surface antigens, hemagglutinin (HA) and
neuraminidase (NA), are the basis of describing the serologic identity of
the influenza viruses using the letters H and N with the appropriate numbers
in the virus designation e.g., H7N2. There are now 15 hemagglutinin and 9
neuraminidase antigens described among the Type A influenza viruses. The
type designation (A, B, or C) is based upon the antigenic character of the M
protein of the virus envelope and the nucleoprotein within the virus
particle. All influenza viruses affecting domestic animals (equine, swine,
avian) belong to Type A, and Type A influenza virus is the most common type
producing serious epidemics in humans. Types B and C do not affect domestic
animals. Classical fowl plague viruses have H7 as one of the surface
antigens but can have different N antigens. It was once believed that all H7
viruses are highly pathogenic fowl plague viruses and that no other avian
influenza viruses could produce a fowl-plague-like disease. When avirulent
AI viruses with the H7 antigens were demonstrated in turkeys in 1971 and
highly virulent AI viruses with the H5 antigen were first found in chickens
in 1959, the necessity for redefining the term fowl plague or using other
terminology became apparent. Because there are highly virulent AI viruses
that possess H antigen other than the H7 and H7 AI viruses that do not
produce clinical fowl plague, an international assembly of avian influenza
specialists proposed that the term fowl plague no longer be used. They
suggested that any AI virus, regardless of its HA designation, meeting
specified virulence requirements in the laboratory be designated highly
pathogenic avian influenza (HPAI). The criteria that serve as the basis for
classifying an AI virus as HPAI has more recently been modified to include
molecular considerations such as the type of amino acids at the cleavage
site of its HA. This chapter will be limited to describing the HPAI and not
the AI viruses of less virulence and pathogenicity.
Host Range Most
avian species appear to be susceptible to at least some of the AI viruses. A
particular isolate may produce severe disease in turkeys but not in chickens
or any other avian species. Therefore, it would be impossible to generalize
on the host range for HPAI, for it will likely vary with the isolate. This
assumption is supported by reports of farm outbreaks where only a single
avian species of several species present on the farm became infected. Swine
appear to be important in the epidemiology of infection of turkeys with
swine influenza virus when they are in close proximity. Other mammals do not
appear to be involved in the epidemiology of HPAI. The infection of humans
with an H5 avian influenza virus in Hong Kong in 1997 has resulted in a
reconsideration of the role of the avian species in the epidemiology of
human influenza
Geographic Distribution
Highly pathogenic avian influenza viruses have periodically occurred
in recent years in Australia (H7), England (H7), South Africa (H5), Scotland
(H5), Ireland (H5), Mexico (H5), Pakistan (H7), and the United States (H5).
Because laboratory facilities are not readily available in some parts of the
world to differentiate Newcastle disease and HPAI, the actual incidence of
HPAI in the world's poultry flocks is difficult to define. It can occur in
any country, regardless of disease control measures, probably because of its
prevalence in wild migratory waterfowl, sea birds and shore birds. Avian
influenza has produced losses of variable severity, primarily in turkeys in
the United States, since the mid-1960's. The disease outbreaks in turkeys in
the United States have been caused by AI viruses with many of the HA
designations. It was in the fall of 1983 that a highly virulent H5 virus
produced severe clinical disease and high mortality in chickens, turkeys,
and guinea fowl in Pennsylvania. This severe disease, clinically
indistinguishable from classical fowl plague, occurred after a serologically
identical but apparently mild virus had been circulating in poultry in the
area for 6 months. Outbreaks of less virulent AI have frequently been
described in domestic ducks in many areas of the world. The AI viruses are
often recovered from apparently healthy migratory waterfowl, shore birds,
and sea birds worldwide. The epidemiologic significance of these isolations
relative to outbreaks in domestic poultry has led to the generally accepted
belief that waterfowl serve as the reservoir of influenza viruses
Transmissions
There is a considerable body of circumstantial evidence to support the
hypothesis that migratory waterfowl, sea birds, or shore birds are generally
responsible for introducing the virus into poultry. Once introduced into a
flock, the virus is spread from flock to flock by the usual methods
involving the movement of infected birds, contaminated equipment, egg flats,
feed trucks, and service crews, to mention a few. Preliminary trapping
evidence indicates that garbage flies in the Pennsylvania outbreak were
sources of virus on the premises of the diseased flocks. Virus may readily
be isolated in large quantities from the feces and respiratory secretions of
infected birds. It is logical to assume, therefore, that because virus is
present in body secretions, transmission of the disease can take place
through shared and contaminated drinking water. Airborne transmission may
occur if birds are in close proximity and with appropriate air movement.
Birds are readily infected via instillation of virus into the conjunctival
sac, nares, or the trachea. Preliminary field and laboratory evidence
indicates that virus can be recovered from the yolk and albumen of eggs laid
by hens at the height of the disease. The possibility of vertical
transmission is unresolved; however, it is unlikely infected embryos could
survive and hatch. Attempts to hatch eggs in disease isolation cabinets from
a broiler breeder flock at the height of disease failed to result in any
AI-infected chickens. This does not mean that broken contaminated eggs could
not be the source of virus to infect chicks after they hatch in the same
incubator. The hatching of eggs from a diseased flock would likely be
associated with considerable risk.
Incubation Period
The incubation period is usually 3 to 7 days, depending upon the isolate,
the dose of inoculum, the species, and age of the bird.
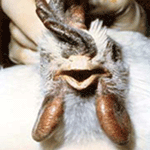 Clinical Signs
Infections of HPAI result in marked depression with ruffled feathers,
in-appetence, excessive thirst, cessation of egg production, and watery
diarrhea. Mature chickens frequently have swollen combs, wattles (See
Pictures at Bottom), and edema surrounding the eyes. The combs are often
cyanotic at the tips and may have plasma or blood vesicles on the surface
with dark areas of ecchymotic hemorrhage and necrotic foci (See Pictures at
Bottom ). The last eggs laid, after the onset of illness, are frequently
without shells. The diarrhea begins as watery bright green and progresses to
almost totally white. Edema of the head, if present, is often accompanied by
edema of the neck. The conjunctivae are congested and swollen with
occasional hemorrhage. Clinical Signs
Infections of HPAI result in marked depression with ruffled feathers,
in-appetence, excessive thirst, cessation of egg production, and watery
diarrhea. Mature chickens frequently have swollen combs, wattles (See
Pictures at Bottom), and edema surrounding the eyes. The combs are often
cyanotic at the tips and may have plasma or blood vesicles on the surface
with dark areas of ecchymotic hemorrhage and necrotic foci (See Pictures at
Bottom ). The last eggs laid, after the onset of illness, are frequently
without shells. The diarrhea begins as watery bright green and progresses to
almost totally white. Edema of the head, if present, is often accompanied by
edema of the neck. The conjunctivae are congested and swollen with
occasional hemorrhage.
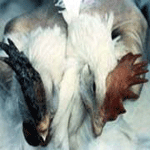 The legs, between the hocks and feet, may have areas
of diffuse hemorrhage (See Pictures at Bottom). Respiratory signs can be a
significant feature of the disease, depending on the extent of tracheal
involvement. Mucus accumulation can vary. It is not unusual in caged layers
for the disease to begin in a localized area of the house and severely
affect birds in only a few cages before it spreads to neighboring cages.
Death may occur within 24 hours of first signs of disease, frequently within
48 hours, or be delayed for as long as a week. Some severely affected hens
may occasionally recover. In broilers, the signs of disease are frequently
less obvious with severe depression, inappetence, and a marked increase in
mortality being the first abnormalities observed. Edema of the face and neck
and neurologic signs such as torticollis and ataxia may also be seen. The
disease in turkeys is similar to that seen in layers, but it lasts 2 or 3
days longer and is occasionally accompanied by swollen sinuses. In domestic
ducks and geese the signs of depression, inappetence, and diarrhea are
similar to those in layers, though frequently with swollen sinuses. Younger
birds may exhibit neurologic signs. The legs, between the hocks and feet, may have areas
of diffuse hemorrhage (See Pictures at Bottom). Respiratory signs can be a
significant feature of the disease, depending on the extent of tracheal
involvement. Mucus accumulation can vary. It is not unusual in caged layers
for the disease to begin in a localized area of the house and severely
affect birds in only a few cages before it spreads to neighboring cages.
Death may occur within 24 hours of first signs of disease, frequently within
48 hours, or be delayed for as long as a week. Some severely affected hens
may occasionally recover. In broilers, the signs of disease are frequently
less obvious with severe depression, inappetence, and a marked increase in
mortality being the first abnormalities observed. Edema of the face and neck
and neurologic signs such as torticollis and ataxia may also be seen. The
disease in turkeys is similar to that seen in layers, but it lasts 2 or 3
days longer and is occasionally accompanied by swollen sinuses. In domestic
ducks and geese the signs of depression, inappetence, and diarrhea are
similar to those in layers, though frequently with swollen sinuses. Younger
birds may exhibit neurologic signs.
Gross Lesions
Birds that die with the per acute disease and young birds
may not have significant gross lesions other than severe congestion of the
musculature and dehydration. In the less acute form, and in mature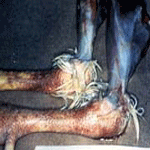 birds, significant gross lesions are frequently observed. They may consist
of subcutaneous edema of the head and neck area, which is evident as the
skin is reflected . Fluid may exit the nares and oral cavity as the bird is
positioned for postmortem examination. The conjunctivae are severely
congested occasionally with petechiation. The trachea may appear relatively
normal except that the lumen contains excessive mucous exudate. It may also
be severely involved with hemorrhagic tracheitis similar to that seen with
infectious laryngo-tracheitis. When the bird is opened, pinpoint petechial
hemorrhages are frequently observed on the inside of the keel as it is bent
back. Very small petechia may cover the abdominal fat, serosal surfaces, and
peritoneum,
birds, significant gross lesions are frequently observed. They may consist
of subcutaneous edema of the head and neck area, which is evident as the
skin is reflected . Fluid may exit the nares and oral cavity as the bird is
positioned for postmortem examination. The conjunctivae are severely
congested occasionally with petechiation. The trachea may appear relatively
normal except that the lumen contains excessive mucous exudate. It may also
be severely involved with hemorrhagic tracheitis similar to that seen with
infectious laryngo-tracheitis. When the bird is opened, pinpoint petechial
hemorrhages are frequently observed on the inside of the keel as it is bent
back. Very small petechia may cover the abdominal fat, serosal surfaces, and
peritoneum,
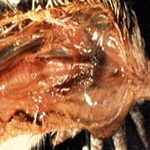 which appears as if it were finely splattered with red paint.
Kidneys are severely congested and may occasionally be grossly plugged with
white urate deposits in the tubules. In layers, the ovary may be hemorrhagic
or degenerated with darkened areas of necrosis. The peritoneal cavity is
frequently filled with yolk from ruptured ova, causing severe airsacculitis
and peritonitis in birds that survive for 7 to 10 days. Hemorrhages may be
present on the mucosal surface of the proventriculus — particularly at the
juncture with the gizzard. The lining of the gizzard peels easily and
frequently reveals hemorrhages and erosions underneath. The intestinal
mucosa may have hemorrhagic areas especially in the lymphoid foci such as
the cecal tonsils. The gross lesions are not distinctly different from those
observed with velogenic viscerotropic Newcastle disease (VVND). The lesions
in turkeys and domestic ducks are similar to those in chickens but may not
be as marked. which appears as if it were finely splattered with red paint.
Kidneys are severely congested and may occasionally be grossly plugged with
white urate deposits in the tubules. In layers, the ovary may be hemorrhagic
or degenerated with darkened areas of necrosis. The peritoneal cavity is
frequently filled with yolk from ruptured ova, causing severe airsacculitis
and peritonitis in birds that survive for 7 to 10 days. Hemorrhages may be
present on the mucosal surface of the proventriculus — particularly at the
juncture with the gizzard. The lining of the gizzard peels easily and
frequently reveals hemorrhages and erosions underneath. The intestinal
mucosa may have hemorrhagic areas especially in the lymphoid foci such as
the cecal tonsils. The gross lesions are not distinctly different from those
observed with velogenic viscerotropic Newcastle disease (VVND). The lesions
in turkeys and domestic ducks are similar to those in chickens but may not
be as marked.
Morbidity and Mortality
The prognosis for flocks infected with HPAI is poor. Morbidity and
mortality rates may be near 100 percent within 2 to 12 days after the first
signs of illness. Birds that survive are usually in poor condition and
resume laying only after a period of several weeks
Diagnosis
Field Diagnosis
Highly pathogenic avian influenza is suspected with any flock where sudden
deaths follow severe depression, inappetence, and a drastic decline in egg
production. The presence of facial edema, swollen and cyanotic combs and
wattles, and petechial hemorrhages on internal membrane surfaces increases
the likelihood that the disease is HPAI. However, an absolute diagnosis is
dependent upon the isolation and identification of the causative virus.
Commercially available type A influenza antigen-capture enzyme linked
immunosorbent assay kits designed for use in human influenza have recently
shown promise as a possible rapid diagnostic test for poultry
Specimens for Laboratory
Specimens sent to the laboratory should be accompanied by a history of
clinical and gross lesions, including any information on recent additions to
the flock. Diagnosis depends upon the isolation and identification of the
virus from tracheal or cloacal swabs, feces, or from internal organs (5).
Specimens should be collected from several birds. It is not unusual for many
of the submitted specimens to fail to yield virus. Swabs are the most
convenient way to transfer AI virus from tissues or secretions of the
suspect bird to brain and heart infusion broth or other cell culture
maintenance medium containing high levels of antibiotics. Dry swabs should
be inserted deeply to ensure obtaining ample epithelial tissue. Trachea,
lung, spleen, cloaca, and brain should be sampled. If large numbers of dead
or live birds are to be sampled, cloacal swabs from up to five birds can be
pooled in the same tube of broth. An alternative technique is to place 0.5
cm3 of each tissue into the broth. Blood for serum should be collected from
several birds. If the specimens can be delivered to a laboratory within 24
hours, they should be placed on ice. If delivery will take longer,
quick-freeze the specimens and do not allow them to thaw during transit
Laboratory Diagnosis
Nine to 11-day-old embryonated chicken eggs are inoculated with swab
or tissue specimens. Avian influenza virus will usually kill embryos within
48-72 hours. If the virus isolated is identified as a Type A influenza
virus, through the AGP or ELISA tests, it is then tested using a battery of
specific antigens to identify its serologic identity (HA and NA type). Sera
from infected chickens usually yield positive antibody tests as early as 3
or 4 days after first signs of disease.
Differential Diagnosis
Highly pathogenic avian influenza is easily confused with VVND,
because the disease signs and postmortem lesions are similar, and may also
be confused with infectious laryngotracheitis and acute bacterial diseases
such as fowl cholera and Escherichia coli. However, in an area where AI is
prevalent, such as during an outbreak, sound presumptive diagnoses can be
made by flock history, signs, and gross lesions.
Treatment
Amantadine hydrochloride has been licensed for use in humans to treat
influenza since 1966. The medication is effective in reducing the severity
of influenza Type A in humans. Experimental evidence indicated possible
efficaciousness in poultry when the drug was administered in drinking water
to reduce disease losses, but drug-resistant viruses quickly emerged,
negating the initial beneficial effects. Thus, the drug is not recommended
for use in poultry
Vaccination
Inactivated oil-emulsion vaccines, although fairly expensive, have been
demonstrated to be effective in reducing mortality, preventing disease, or
both, in chickens and turkeys (7). These vaccines may not, however, prevent
infection in some individual birds, which go on to shed virulent virus. More
economical viable vaccines prepared using naturally avirulent or attenuated
strains have the disadvantage of the possible creation of reassortant
influenza viruses with unpredictable characteristics. These reassortants
could result when a single host bird is simultaneously infected with both
the vaccine and another AI virus. Owing to the segmented nature of the
influenza virus genome, a reassortment of genetic material can readily
occur, creating new influenza viruses. The basic drawback to any vaccine
approach for the control of HPAI is the large number of HA subtypes that can
cause the disease. Because there is no cross-protection among the 15 known
HA subtypes, either a multivalent vaccine will be needed or vaccination
postponed until the prevalent disease-causing subtype in the area is
identified. A recombinant fowl pox virus vaccine containing the gene that
codes for the production of the H5 antigen has recently been licensed. The
use of a recombinant insect virus containing the gene for either the H5 or
H7 antigen has been used to make these vaccine proteins in insect cell
cultures
Control and Eradication
Wild birds and their excreta should be considered a major source of
avian influenza. Preventing direct contact with free-flying birds and
protecting domestic poultry from contact with the feces of wild birds is an
important way to prevent avian influenza. Live bird markets have been an
important source of avian influenza. It is important to avoid live markets,
educate employees about the dangers posed by these markets, and prevent the
spread of disease from these markets to your flock by preventing any
contact. Infected birds shed virus in saliva, nasal secretions and feces in
the first two weeks of infection. Four weeks after infection, virus can no
longer be detected. Hence, prevention is best accomplished by preventing
contact between newly infected and susceptible birds. Biosecurity is a first
line of defense. Avian influenza can be spread from infected birds through
the transfer of feces especially on contaminated equipment and clothing.
Controlling the traffic between infected and uninfected birds is essential.
Cleaning and
disinfection Influenza viruses are very sensitive to most detergents
and disinfectants. They are readily inactivated by heating and drying.
However, flu viruses are well-protected from inactivation by organic
material and infectious virus can be recovered from manure for up to 105
days. Complete removal of all organic material is part of any effective
disinfection procedure. Contaminated houses are heated for several days to
inactivate virus. Organic material is removed followed by complete cleaning
and disinfection of all surfaces. Contaminated litter and manure is
problematic and should be composted or buried to ensure that it does not
spread infectious virus.
Frequently asked
questions 1. Are the flu viruses of human and birds the same? In most
cases, the influenza viruses that infect birds do not infect humans and vice
versa. However, in Hong Kong in 1997, a unique avian influenza virus
infected both chickens and humans. This is the only time an avian influenza
virus has ever been transmitted directly from birds to humans. This appears
to have been a totally unique occurrence. The World Health Organization
continuously monitors human influenza viruses isolated from cases all over
the world. No avian viruses have been found infecting humans since 1997.
2. What are the
risks of getting avian influenza from waterfowl? Avian influenza virus
infections are widespread in wild birds, especially ducks. Migrating
waterfowl are a significant source of avian influenza viruses especially in
the major flyways. Turkeys on open ranges in Minnesota, a state in the major
flyway for migrating ducks, frequently experience avian influenza problems.
But the prevalence of avian influenza in turkeys has been high in some years
and minimal in others. The reason why influenza viruses come and go is not
known. The risk to susceptible birds from contact with waterfowl must be
considered very high although it may vary from year to year for unknown
reasons.3 Why
can't I prevent infection by vaccinating my flocks? Vaccines effectively
prevent clinical signs of influenza infections in many species including
poultry. However, the vaccines are not cross-protective for the 15 virus
subtypes that can infect poultry. Since there is no way to predict which
type will infect a flock, vaccines are generally not practical to prevent
infections. 4.
What should I do if I suspect avian influenza in my birds? You should
contact your veterinarian if you observe any of the signs of avian
influenza, especially if they are accompanied by a drop in feed consumption
and/or a significant drop in egg production. Because the signs of avian
influenza are so variable, it is important to get the help of an expert for
diagnosis.
Avian Influenza Type A influenza viruses can infect several animal species,
including birds, pigs, horses, seals and whales. Influenza viruses that
infect birds are called “avian influenza viruses.” Birds are an especially
important species because all known subtypes of influenza A viruses
circulate among wild birds, which are considered the natural hosts for
influenza A viruses. Avian influenza viruses do not usually directly infect
humans or circulate among humans. Influenza A viruses can be divided into
subtypes on the basis of their surface proteins — hemagglutinin (HA) and
neuraminidase (NA). There are 15 known H subtypes. While all subtypes can be
found in birds, only 3 subtypes of HA (H1, H2 and H3) and two subtypes of NA
(N1 and N2) are known to have circulated widely in humans. Avian influenza
usually does not make wild birds sick, but can make domesticated birds very
sick and kill them. Avian influenza A viruses do not usually infect humans;
however, several instances of human infections and outbreaks have been
reported since 1997. When such infections occur, public health authorities
monitor the situation closely because of concerns about the potential for
more widespread infection in the human population.
Avian Influenza
Infections in Humans: Confirmed instances of avian influenza viruses
infecting humans since 1997 include:
1997: In Hong Kong,
avian influenza A (H5N1) infected both chickens and humans. This was
the first time an avian influenza virus had ever been found to transmit
directly from birds to humans. During this outbreak, 18 people were
hospitalized and 6 of them died. To control the outbreak, authorities killed
about 1.5 million chickens to remove the source of the virus. Scientists
determined that the virus spread primarily from birds to humans, though rare
person-to-person infection was noted.
1999: In Hong Kong,
cases of avian influenza A H9N2 were confirmed in 2 children. Both patients
recovered, and no additional cases were confirmed. The evidence suggested
that poultry was the source of infection and the main mode of transmission
was from bird to human. However, the possibility of person-to-person
transmission remained open. Several additional human H9N2 infections were
reported from mainland
China in 1998-99.
2003: Two cases of avian influenza A (H5N1)
infection occurred among members of a Hong Kong family that had traveled to
China. One person recovered, the other died. How or where these 2 family
members were infected was not determined. Another family member died of a
respiratory illness in China, but no testing was done. No additional cases
were reported.
2003: Avian influenza A
(H7N7) infections among poultry workers and their families were
confirmed in the Netherlands during an outbreak of avian flu among poultry.
More than 80 cases of H7N7 illness were reported (the symptoms were mostly
confined to eye infections, with some respiratory symptoms), and 1 patient
died (in a veterinarian who had visited an affected farm). There was
evidence of some human-to-human transmission.
2003: H9N2
infection was confirmed in a child in Hong Kong. The child was hospitalized
but recovered
Characteristics of Avian Influenza in Birds: Certain water
birds act as hosts of influenza viruses by carrying the virus in their
intestines and shedding it. Infected birds shed virus in saliva, nasal
secretions and feces. Avian influenza viruses spread among susceptible birds
when they have contact with contaminated nasal, respiratory and fecal
material from infected birds; however, fecal-to-oral transmission is the
most common mode of spread. Most influenza viruses cause no symptoms, or
only mild ones in wild birds; however, the range of symptoms in birds varies
greatly depending on the strain of virus and the type of bird. Infection
with certain avian influenza A viruses (for example, some H5 and H7 strains)
can cause widespread disease and death among some species of wild and
especially domesticated birds such as chickens and turkeys. Symptoms of
Avian Influenza in Humans The reported symptoms of avian influenza in humans
have ranged from typical influenza-like symptoms (e.g., fever, cough, sore
throat and muscle aches) to eye infections, pneumonia, acute respiratory
distress, viral pneumonia, and other severe and life-threatening
complications. Antiviral Agents for Influenza Studies to date suggest that
the prescription medications approved for human influenza strains would be
effective in preventing avian influenza infection in humans, however,
sometimes flu strains can become resistant to these drugs and so they may
not always be effective.
Potential for an Influenza Pandemic: All influenza
viruses can change. It is possible that an avian influenza virus could
change so that it could infect humans and could spread easily from person to
person. Because these viruses do not commonly infect humans, there is little
or no immune protection against them in the human population. If an avian
virus were able to infect people and gain the ability to spread easily from
person to person, an “influenza pandemic” could begin.
Background on Pandemics
An influenza pandemic is a global outbreak of influenza and occurs when a
new influenza virus emerges, spreads, and causes disease worldwide. Past
influenza pandemics have led to high levels of illness, death, social
disruption and economic loss. There were 3 major pandemics in the 20th century.
All of them spread worldwide within 1 year of being detected. They are:
1918-19, "Spanish flu,"
[A (H1N1)], caused the highest number of known flu deaths: more than
500,000 people died in the United States, and 20 million to 50 million
people may have died worldwide. Many people died within the first few days
after infection and others died of complications soon after. Nearly half of
those who died were young, healthy adults.
1957-58, "Asian flu," [A
(H2N2)], caused about 70,000 deaths in the United States. First
identified in China in late February 1957, the Asian flu spread to the
United States by June 1957.
1968-69, "Hong Kong
flu," [A (H3N2)], caused approximately 34,000 deaths in the United
States. This virus was first detected in Hong Kong in early 1968 and spread
to the United States later that year.
Type A (H3N2) viruses
still circulate today. Once a new pandemic influenza virus emerges
and spreads, it typically becomes established among people and circulates
for many years. The U.S. Centers for Disease Control and Prevention and the
World Health Organization conduct extensive surveillance programs to monitor
the occurrence of influenza activity worldwide, including the emergence of
potential pandemic strains of influenza virus. For this reason, public
health concerns about the present H5N1 situation must be given the highest
priority when weighing the immediate and measurable economic losses in
animals against possible yet unpredictable consequences for humans. Several
other diseases in animals can be transmitted to humans. Experience with such
diseases, known as “zoonoses”, has shown that strict measures on animal
health, imposed by the need to protect human health, helped rebuild consumer
confidence. Recent experience has also shown that measures for the control
of zoonotic diseases, aimed at halting further spread in animals and
minimizing economic losses, need to be closely coordinated with measures
that minimize the longer-term risks to human health. In the present
situation, measures aimed at eliminating the disease in poultry will also
reduce the presence of the virus in the environment and thus reduce
opportunities for human exposures and infections. These measures must be
carried out urgently, giving highest priority to the protection of human
health. Previous outbreaks of highly pathogenic avian influenza associated
with human infections occurred in areas, such as Hong Kong and the
Netherlands, with industrial poultry production and well developed health
and agricultural infrastructures. Even so, elimination of infection in
poultry was a complex, difficult, and costly undertaking. Both outbreaks
were eventually controlled through immediate culling of infected flocks,
quarantine and disinfection of farms, strict biosecurity, restrictions on
the movement of animals, and compensation for farmers. The present situation
is different. Control of outbreaks of highly pathogenic avian influenza is
known to be especially difficult in areas where poultry range freely. In
several affected countries, up to 80% of the total poultry population is
raised in small backyard farms. Most rural families keep a small free-range
flock. Given these features of the present situation there is potential that
the H5N1 virus could become established in bird populations in this
geographical region and possibly spread to other parts of the world. This
was one of several conclusions reached during a joint FAO/OIE/WHO technical
consultation on the control of avian influenza, held in Rome from 3–4
February. No single blueprint for control in animals, and thus reduction of
risks for humans, is available. Over the past four decades, only 18
outbreaks of highly pathogenic avian influenza, most caused by strains other
than H5N1, have occurred throughout the world. Existing evidence will not
suffice to provide universally applicable recommendations for a rapid and
effective response in affected countries. Control measures must be tailored
to each country’s unique epidemiological situation and unique capacity, with
health and agricultural sectors working hand-in-hand. Agricultural
authorities face the immediate challenge of rapidly eliminating the H5N1
reservoir in poultry. Authorities in all affected countries need to work
together in a coordinated way Transparency in reporting of human and animal
disease is absolutely essential. Despite the uncertainties, experts fully
agree that immediate culling of infected and exposed birds is the first line
of defense for both the protection of human health and the reduction of
further losses in the agricultural sector. Other measures, such as the
vaccination of healthy flocks, may play a supportive role in some cases when
undertaken in conjunction with measures for preventing further spread of
infection. WHO has repeatedly stressed the need to ensure that culling is
carried out in a way that does not fuel more human cases. and that
vaccination of poultry should not lead to the dropping of vigilance or
compromise other necessary control measures. In responding to the situation,
WHO emphasises three strategic goals: to avert an influenza pandemic, to
control the present human outbreaks and prevent further spread, and to
conduct the research needed for better preparedness and response, including
the immediate development of a new vaccine for humans against H5N1. WHO has
issued a series of technical guidelines aimed at minimizing the risk of
further human cases and facilitating a coordinated international response.
Highly pathogenic avian influenza is categorized by OIE as a “list A”
disease. List A includes transmissible diseases “which have the potential
for very serious and rapid spread, irrespective of national borders, which
are of serious socio–economic or public health consequence and which are of
major importance in the international trade of animals and animal products.”
One example is the spread of bovine spongiform encephalopathy, or “mad cow
disease”, in cattle, which led to the emergence of a rare yet invariably
fatal new disease in humans.
|
